
Our fields of
SPECIALTY

We specialise in translating regulatory documentation, decrees and authorisations from regulatory bodies, Certificates of Pharmaceutical Product (CPPs), Manufacturing and Importation Authorisations (MIA), GMP certificates, Certificates of Homeopathic Product (CPOs) and Pharmacovigilance Inspection Reports.
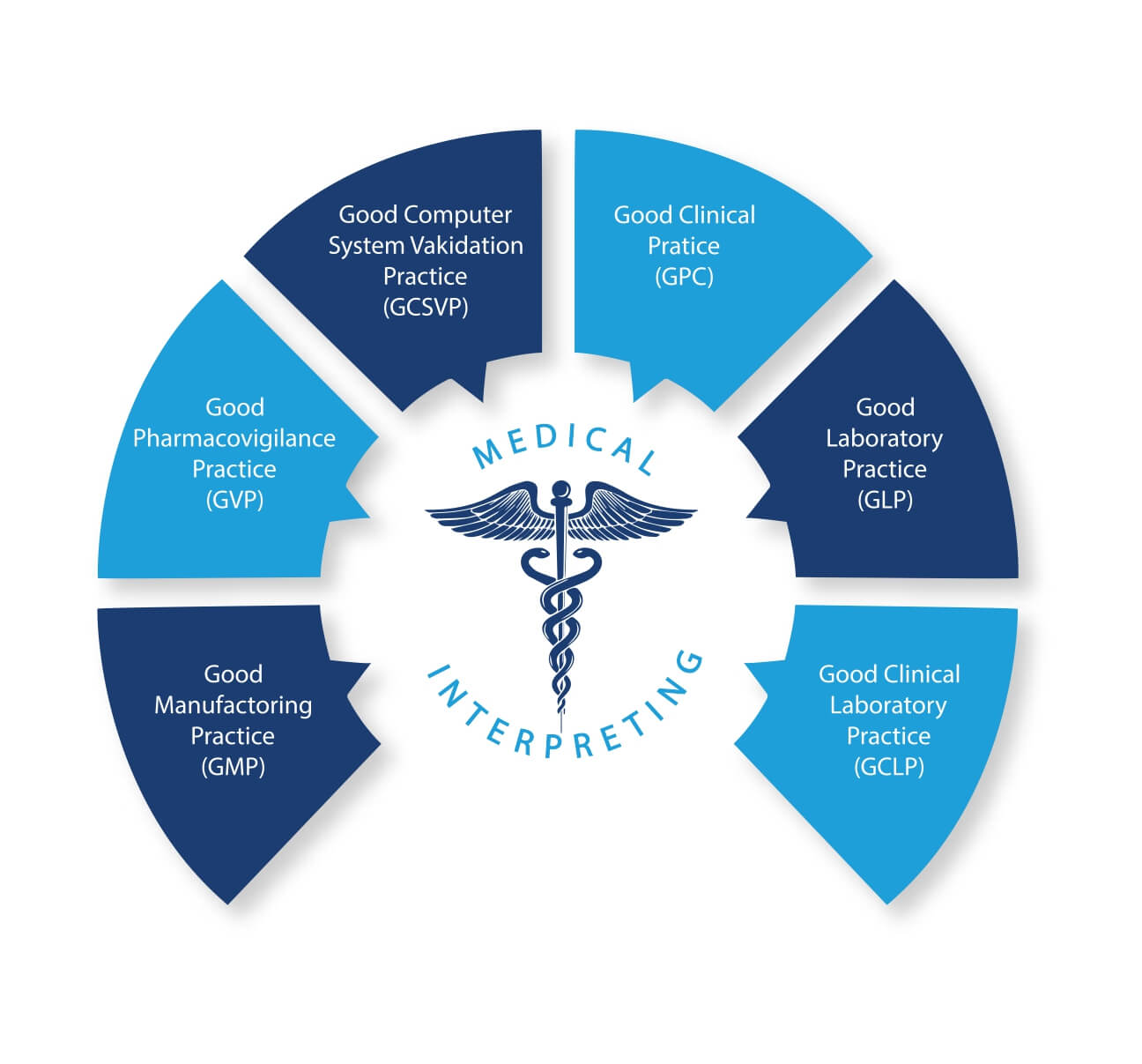
We specialise in medical interpreting and translation, specifically within the realms of clinical trial protocols and GCP regulatory regulations.
Our specialised knowledge encompasses multiple fields of medicine, including Haematology, Endocrinology, Oncology, Cardiology, Immunology, Neurology, Anatomy, Biotechnology, Pneumology, Life Science, and numerous other specialisations. With a well-established and comprehensive expertise, we excel in offering language support during periodic Good Manufacturing Practices (GMP) compliance inspections conducted at manufacturing sites. These inspections are meticulously coordinated and managed by Regulatory Authorities, both Italian and foreign, supported by accomplished interpreters in this domain.
We specialise in translating Product Monographs, Product Information (Summaries of Product Characteristics, Package Leaflets, Labelling and the Blue-Box), Study and Clinical Trial Protocols, Informed Consent Forms, Adverse Event Reports and Scientific Articles. We excel for our extensive experience in the Linguistic Review Process for Product Information, encompassing the Summary of Product Characteristics, Package Leaflet, and Labelling. The key objective of this review is to confirm that the information is compliant with European Medicines Agency (EMA) guidelines, complies with templates provided by competent authorities such as the EMA Working Group on Document Quality Review (QRD), and meets country-specific regulatory requirements.

We specialise in translating preclinical and clinical documentation, Quality, Safety & Efficacy Documentation, Expert Reports, Package Leaflets, Summaries of Product Characteristics and Labelling.
We specialise in translating Clinical Product Synopses, Evaluation Studies, Patient Information Leaflets, Informed-Consent Forms and Scientific Reports.


